There has been a lot of press in the last week about a new way of genetically testing embryos to improve fertility and reduce the risk of miscarriage. This is because embryology labs have established a new method to be able to assess the chromosomes of embryos. They are satisfied that their validations mean it is accurate enough to now offer to patients as part of their IVF cycle during fertility treatment. I want to explain the pros and cons of using this new method of genetic testing embryos to couples under my care and to compare it to our current standard method, as I believe it is likely to become the main way of testing embryos in IVF cycles in the future.

What does preimplantation genetic testing mean in IVF?
When couples undertake IVF or ICSI fertility treatment, one of the main reasons that embryos either don’t implant or go on to miscarry is because they have either too many or too few chromosomes. Chromosomes are structures made up of DNA. We make all the proteins in our bodies from gene codes embedded in our DNA. So, if all the DNA from one chromosome is missing or is in excess, we will have either too much or not enough of the proteins encoded by that chromosome. Most of the time when the chromosome numbers are not right (are not balanced), an embryo simply won’t implant, however sometimes the embryo will create a pregnancy but the foetus won’t form properly so a miscarriage occurs and occasionally if the chromosome is very small ( like chromosome 21) an embryo can form a live baby with a condition like Down’s Syndrome.
Which couples are at risk of having embryos with chromosome problems?
Maternal age has the biggest influence on the number of chromosomes present in the embryo. For women, all our eggs are stored in the ovary when we are an embryo, and these eggs are stored part way through a cell division. This means all the chromosomes in the eggs are lined up on fine strands called spindles. For everyone, as we age there is more time for the cells in our body to be damaged. For most cells, if damage occurs (from pollution, smoking, general wear and tear etc) the cell will either repair itself or the cell dies. Eggs can’t do this. This means that on average when a woman is 34, about 40% of her eggs will have damage, by 40years, about 50% will have damage, by 43 years, 70% will have damage and by 45 years 90-95% of eggs will be damaged.
Damaged sperm can also increase the risk of embryos with chromosome problems. Reducing factors that cause DNA damage (see blog on Improving Fertility by Creating a Healthy Environment for Sperm Eggs and Embryos) can improve embryo quality and reduce miscarriage rates.
Rarely couples have a genetic condition that gives them an increased risk of having embryos with an unbalanced set of embryos (a translocation). Genetic testing can reduce the time to conception of a healthy pregnancy and reduce the chance of miscarriage.


Why do couples choose to genetically test their embryos?
- Sometimes couples have delivered a child with a chromosome disorder or have had miscarriages or pregnancy losses and want to minimise the risk of chromosomal problems affecting a further pregnancy.
- Women under 38 have a higher chance of creating embryos with normal chromosomes that older women so it is more relevant to discuss and consider this option when the female partner is in their late 30s and into their 40s.
- When sperm quality or DNA damage might increase the risk of a chromosome problem.
- When a genetic condition like a translocation might lead to more embryos with chromosome problems.
- If there are several embryos, particularly when maternal age is higher, testing the embryo’s chromosomes in the lab (rather than in the mother’s body) may reduce the number of frozen embryo transfer cycles that are not going to result in a livebirth. Transferring only genetically normal embryos may save time and money.
Why is genetic testing a breakthrough and how is it beneficial?
Understanding Standard Chromosome testing.
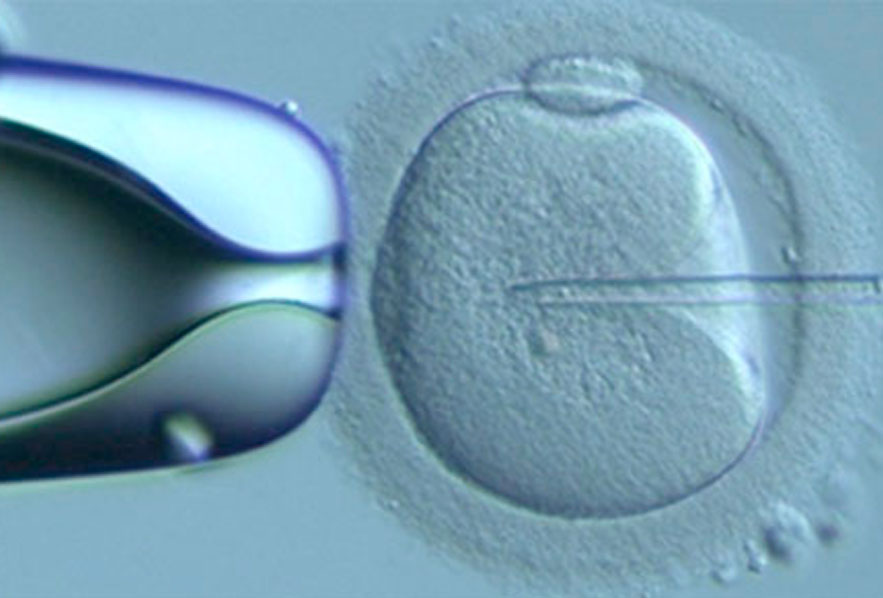
Firstly, I need to explain the way we currently test embryos and some of the uncertainties of this test. Our standard method of testing embryos is to biopsy a few cells from an embryo created during IVF or ICSI treatment. We used to do this at day 3 of embryo development in the lab, but now know that the test is much better if we do it on Day 5 or 6 when there are a lot more cells and we can amplify and test the DNA from 4-5 cells (rather than 1 or 2).
We make a small hole in the shell around the embryo (zona pellucida) and if the embryo grows well it will start to hatch out of this shell by Day 5 or 6. As long as the part that is hatching is not the baby part of the embryo (the inner cell mass) and some of the cell are poking out of the shell, we can do the testing. We use a micro-lazer to remove 4-5 cells. The DNA from these cells is then extracted and amplified. Most labs now sequence the DNA, identifying the code embedded in the DNA. If there is a chromosome problem, there will be too much DNA or not enough DNA from that chromosome seen in the analysis. The embryos have to be frozen (and we have very good freezing methods now) to give us time to analyse the chromosomes before the embryo is replaced in the womb. This means a tested embryo has to be replaced in a frozen embryo transfer cycle.
One difficulty is quite a lot of the time the embryo is not quite big enough for cells to push out from the shell or the baby part pokes out first and a biopsy could potentially damage the foetus. Sometimes we can’t extract the DNA from the cells or not enough DNA amplifies to be able to test. In these situations, the chromosomes can’t be tested, and the number of chromosomes remains unknown. This is a relatively common occurrence and we have to make a decision as to whether to transfer the embryo without the genetic information.
Another difficulty is that as an embryo grows, they sometimes misplace a chromosome in later cell divisions. The embryo has corrective mechanisms to remove these incorrectly divided cells as it develops, and continuously does this. If by chance one or two of the 4-5 cells that are biopsied have an incorrect number of chromosomes and were going to be ‘corrected’ by the otherwise normal embryo, we get an answer that indicates the embryo is a mosaic (not all the cells have the same number of chromosomes). It can be very hard to know whether to replace embryos with a mosaic result.
One last concern about taking cells away from embryos is how stressful the biopsy might be for the embryo. It is another invasive procedure the embryo has to go through, when it will also have to be frozen and thawed.

How might genetic testing address these problems?
In genetic testing, the embryo has to be cultured on its own so the DNA it secretes can be extracted and amplified from the surrounding media. This means that even embryos that haven’t hatched can be tested, although they may have to be cultured for a slightly longer time to allow enough DNA to collect in the media. Thus, the biggest advantage of this technique is that genetic information can be obtained about embryos that we couldn’t test before. One aspect however is that only embryos that have been fertilised by injecting the sperm into the egg (ICSI) can be tested as if fertilisation has occurred by placing the sperm and egg together in the media (IVF), we could end up measuring the sperm’s DNA not the embryo’s and have a false result. The accuracy of genetic testing has been validated and papers are in publication.
Although unproven as yet, testing is more likely to represent more cells of the embryo than the few that are biopsied currently. Experiments are currently being designed to determine how representative the DNA in the media is of all the cells in an embryo.
If an embryo doesn’t have to be biopsied it does not have to spend energy recovering from this procedure. This must be less stressful for the embryo, which is particularly relevant when embryos are fragile or when there are not many of them.